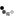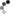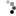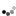 Loading... Please wait...
Loading... Please wait...Free Shipping on orders over $500
- Home
- Medical Apparel
- 3M 1860 Surgical Mask
Categories
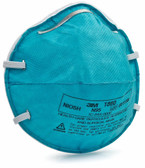
3M 1860 Surgical Mask
Product Description
3M 1860 Surgical Mask and Particulate Respirator
During the public health emergency of the COVID-19 pandemic, the 3M N95 1820 “industrial” respirators may be used by healthcare professionals according to the U.S. FDA’s Emergency Use Authorization (EUA). 3M Particulate Respirator 8210 is not cleared by the U.S. FDA as a surgical mask, as a surgical mask and is not considered fluid resistant, therefore a face shield must be worn during use.
This healthcare respirator is designed to help provide respiratory protection for the wearer. It meets CDC guidelines for Mycobacterium tuberculosis exposure control. As a disposable particulate respirator, it is intended to help reduce wearer exposure to certain airborne particles including those generated by electrocautery, laser surgery, and other powered medical instruments. As a surgical mask, it is designed to be fluid resistant to splash and spatter of blood and other infectious materials.
- Widely used 3M 1860 Surgical Mask
- NIOSH Approved: N95
- Meets CDC guidelines for Mycobacterium tuberculosis exposure control
- FDA Cleared for use as surgical mask
- >99% BFE (Bacterial Filtration Efficiency) according to ASTM F2101
- Fluid resistant according to ASTM F1862
- Helps protect against certain airborne biological particles
- Respirator contains no components made from natural rubber latex
- Collapse resistant cup shape design
- Braided headbands, cushioning nose foam, and light weight construction for comfortable wear
- 20 Surgical Masks per box
- 6 Boxes per case
- Manufacturer: 3M Health Care
- Manufacturer's Product ID: 1860
WARNING: These respirators help reduce exposures to certain airborne contaminants. Before use, the wearer must read and understand the User Instructions provided as a part of the product packaging. In the U.S., a written respiratory protection program must be implemented meeting all the requirements of OSHA 1910.134 including training, fit testing and medical evaluation. In Canada, CSA standards Z94.4 requirements must be met and/or requirements of the applicable jurisdiction, as appropriate. Misuse may result in sickness or death. For proper use, see package instructions, supervisor, or call 3M OH&ESD Technical Service in USA at 1-800-243-4630 and in Canada at 1-800-267-4414.