 Loading... Please wait...
Loading... Please wait...Free Shipping on orders over $500
Categories
The Benefits of Biological Indicators in the Medical Sterilization Process
Posted on 01/27/2019 10:15:56

Sterilization is critical for dental practices, hospitals, surgery centers, and many more medical facilities around the world because sterilization provides protections to the medical staff and the patient. Medical instruments, equipment, and implants must be completely sterile before they can be used. The sterilization of medical equipment is critical to the functioning of our modern healthcare system at every level. Sterilization prevents cross contamination and improves patient outcomes.
Biological indicators monitor the sterilization process and equipment to ensure the process and equipment are functioning as required to sterilize the medical equipment. For this reason, biological indicators are one of the most critical components of the sterilization process.
Biological indicators are generally considered the highest standard for monitoring the sterilization process. Biological indicators use bacterial spores that are enclosed in a smaller container that is placed inside the sterilizer. After going through the sterilization process the biological indicators are incubated to determine if any bacterial spores survived. If all bacterial spores are eliminated the indicator shows the sterilization process was effective and the medical items in the sterilizer should have zero or significantly fewer microorganisms on them.
Biological Indicators in the Medical Sterilization Process
- How Do You Define the Sterilization Monitoring Process?
- How Does Chemical Monitoring Work?
- How Does Mechanical Monitoring Work?
- How Does Biological Monitoring Work?
- What Exactly is a Biological Indicator for Sterilization?
- What Spores are Used to Make Biological Indicators in Steam Sterilization?
- Why Should We Be Using Biological Indicators?
Medicine would not have entered the modern age were it not for our ability to kill the pathogens that create health problems for the entire population. Because it is such an important facet of our medical system, strong quality assurance procedures are required to monitor the sterilization process.
How Do You Define the Sterilization Monitoring Process?
Sterilization, sometimes referred to as decontamination, is the 6-log reduction of living organisms that occurs when there is a million-fold reduction. In other words, sterilization kills microscopic bacteria and pathogens in order to prevent the spread of human disease.
There are multiple processes used for sterilization; the most common are ethylene oxide and steam. With regards to steam sterilization, the following agents are most often used: gas plasma, hydrogen peroxide, and liquid sterilization. In recent years, the sterilization process has become more efficient than ever with a chemical sterilization agent like glutaraldehyde solution and CIDEX OPA Solution, a high-level disinfectant solution . Sterilization methods vary, but all serve the same function: to eliminate any bacteria and other microorganisms on the surface. As one would expect, all forms of sterilization must be monitored to make sure that all of the potential pathogens are successfully being eradicated.
The sterilization cycle can be influenced by all sorts of factors. The experience of the person operating the machinery can come into play. In fact, human error is the cause of the majority of sterilization load failures. Load preparation can impact the cycle, as can the mechanical condition of the sterilizer. The sterilization process must be monitored to ensure reliability and efficacy, and processing staff must take immediate action if any alarm bells are raised during sterilizing. The items needed for sterilization should be purchased from a reliable medical and surgical supply store known to carry quality products. Healthcare facilities, bound to quality assurance standards, typically use three types of indicators to monitor sterilization: chemical, mechanical and biological.
The biological indicator is considered the gold standard of sterilization monitoring. When used routinely as part of a strategy for long-term monitoring, biological indicators offer assurance in the sterilization process. However, many biological indicators require a lengthy incubation time and therefore can't be reasonably used for every cycle (an exception to this would be the rapid read-out indicator). Biological indicators are recommended used on a weekly basis and because of this, mechanical and chemical methods are part of the sterilization assurance approach.
Let's take a look at the other forms of sterilization monitoring in order to better understand the important role that biological indicators play.

How Does Chemical Monitoring Work?
Chemical monitoring describes the process of using special indicators (like strips, tabs, and tapes) which contain chemicals that produce a change in color as a response to high temperatures (including dry heat). In sterilization, this is commonly referred to as a Class 5 integrating indicator. Chemical indicators must be used for every package and every cycle to ensure that the contents have been properly sterilized. Chemical indicators have shown to be very reliable in verifying that the conditions needed for sterilization have been met, and the results appear on the indicator immediately following the cycle (a huge benefit for sterile processing staff).
Chemical indicators also serve as a label to verify that the items have been processed and are safe for the patient. If a medical professional were to pick up an instrument packet only to find that the color change didn't occur, this signifies a failed test. Under no circumstances should those instruments be used.
How Does Mechanical Monitoring Work?
Mechanical (physical) monitoring describes the observation of the components of the sterilizer as part of routine quality control activities. For each load, the user checks the gauges, displays and unit cycle timers. Variations in these readings might be the first indication that something is wrong with the sterilizer.
How Does Biological Monitoring Work?
Biological indicators are used in the medical sterilization setting to measure the effectiveness of the sterilization process and guarantee the lethality of the cycle. Biological indicators are sometimes referred to as living indicators. This term is fairly self-explanatory: Biological or living indicators are living organisms that are sensitive to change and can provide us with feedback about the state of their direct environment. An example often cited to help explain the concept of bioindicators is that of a plant called lichens and their use in measuring air pollution. Lichen is highly sensitive to pollutants in the air, like sulfur dioxide, and will cease to grow if levels of air pollutants rise. In this way, lichens are a living, naturally-occurring biological indicator of air quality.
What Exactly is a Biological Indicator for Sterilization?
In the medical sterilization setting, biological indicators aren't plants; they are spores of bacteria. And these aren't just any old spores-- these are super powerful bacterial spores known for their resistance to the sterilization process. The biological indicator, also known as a spore strip, consists of a special type of material upon which spores and endospores of bacteria have been applied. The spore strips contain one million bacterial spores on average. Usually placed within something known as a process challenge device, the spore-covered material is processed right along with the other items. Once the cycle is complete, the spore strip is placed into either a vial or glass ampule, sealed, and incubated for a defined period of time. Once the indicated time has passed, the results of the biological indicator test are checked to see if any spore growth has occurred. If it has, that means that at least one spore survived the sterilization process, making the test a failure. Today, we have access to new rapid-readout biological indicators that use enzymes to evaluate the strip for any lingering spores, eliminating the need for an incubation period.
Biological indicators are considered a medical and surgical supply item. One of the biological indicators most commonly seen in the clinic supply cabinet is the 3M 1261 Attest Biological Indicator s and the 3M Attest 1292 Rapid Readout Biological Indicators . Biological indicators are used in conjunction with a process challenge device to monitor the efficacy of the steam sterilizer, or autoclave, along with rooms, biological safety cabinets, and isolators. The process challenge device that contains the biological indicator (and Class 5 integrating indicator) need to go into the most challenging, hard-to-reach spot. For example, this would be the lowermost shelf close to the drain in the sterilizer chamber or the bottom of a trash can in a room.
After the sterilization cycle has run through, the process challenge device is removed and the biological indicators set aside for test results. At this point, the biological indicator method you are using dictates how it will be processed. The rapid read-out indicator uses reactive enzymes to provide a reading of results within one to three hours, and there is no need for incubation (although some may choose to incubate as a double check). Rapid readout indicators are considered equivalent to standard biological indicators. If an early readout biological indicator is not used, it will need to be dropped into a medium for growth with the use of the aseptic technique for incubation.
After a period of time (usually one to three days), the results of the test appear on the biological indicator incubator and show a pass or fail test score result for that cycle. Results may be compared to strips left untreated by spores in order to reduce any uncertainty in the results. Any time a positive spore test comes through, the sterilizer must be immediately checked for malfunction and challenged. If a second positive test occurs, the sterilizer must be taken offline and removed from the central processing area until it has been serviced.

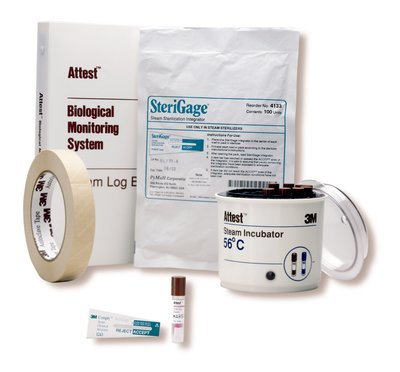
For medical facilities looking for a starter kit, 3M makes the 3M Attest Monitoring Starter Kit for Steam. This kit includes the essentials needed to start monitoring the steam sterilization process. The starter kit includes: (1) Roll of 3M 1322 Indicator Tape, (1) Box of 3M 1262P Biological Indicators, (1) 3M 116 Incubator, (1) Bag of 3M 1243B Chemical Integrators, (1) 3M 1266 Log Book, and (1) Wall chart.
What Spores are Used to Make Biological Indicators in Steam Sterilization?
Biological indicators used for evaluating a steam sterilizer (also known as an autoclave) contain spores from Geobacillus stearothermophilus, Bacillus atrophaeus or Bacillus subtilis. These spores are used as they are known to be particularly resistant to the lethal effects of moist heat. Let's take a look at each of these spores.
Geobacillus Stearothermophilus
A thermophile (heat-loving) type of bacterium that lives in soil. The strains of Geobacillus stearothermophilus used to validate sterilization are capable of aerobically oxidizing carbon monoxide. In recent years, a strain modified with a fluorescent tag has emerged that will give a reading of results in one-tenth the time required. Fun fact: Geobacillus stearothermophilus is responsible for causing food to spoil.
Bacillus Atrophaeus
Bacillus atrophaeus is a species of black-pigmented bacteria frequently used in the field of biomedicine. Fun fact: Bacillus atrophaeus is one of the bacterial strains used in the US biological weapon program that was discontinued in 1969.
Bacillus Subtilis
Sometimes called the hay bacillus or grass bacillus, this species is found in soil and the digestive tracts of humans and other mammals. Bacillus subtilis is an endospore with a rod-like shape and a tough outer shell that enables it to tolerate the most extreme of environments. Fun fact: A sub-type of Bacillus subtilis is used in the fermentation process to make a sticky, strongly-flavored Japanese dish called natto.
Why Should We Be Using Biological Indicators?
The Centers for Disease Control recommends that the sterilization process be monitored with biological indicators on a weekly basis, at a minimum. An expert from the Association for the Advancement of Medical Instrumentation (AAMI) describes biological monitoring as "the cornerstone of your sterilization quality assurance program." Biological indicators tell us whether the sterilization cycle has adequately killed microbial contaminants in a proof-positive way that cannot be achieved by chemical and mechanical monitoring alone.
For sterilizers that process several loads each day, daily use of biological indicators is one of the most effective ways to spot early malfunctions or errors, greatly reducing patient risk. Central processing units and other healthcare settings are wise to incorporate the use of biological indicators as standard practice. Truly, no other indicators come close to the accuracy of spore strips for directly validating the lethality of the sterilization process.
The Importance of Biological Indicators
Biological indicators are an integral and necessary part of the three-pronged approach to monitoring the sterilization process of equipment and surgical tools . Mechanical indicators are usually the most obvious and routine of the indicators. Chemical indicators measure the environment inside the autoclave (or other disinfection unit) to ensure that the conditions lethal to microbiology have been met, like a high temperature. Biological indicators provide the only way to actually know, in terms of proof, that the bacteria has been eradicated by the sterilization process.
Biological indicators, also known as spore strips, should be used at least once per week to challenge the sterilization cycle. Standard biological indicators don't provide results in real time as they require a 24-48-hour incubation period. For those seeking a more immediate option, there are types of biological indicators that can provide results in one hour, such as this 3M Attest 1291 Rapid Readout Biological Indicators. Whatever type of biological indicator you choose for your clinic, by using these indicators you are helping to lower the risk of infection-- and reducing costs associated with fighting infection-- simply by following the standard of care for sterile processing.

Many companies manufacture biological. 3M Attest biological indicators are used in a wide range of medical and dental facilities around the world. While the 3M Attest biological indicators are widely used, the Crosstex biological indicators are also widely used. The Crosstex ConFirm 24 Steam biological indicators can be used in most incubators including most 3M Attest incubators.
Combination Test Packs
Some product lines such as 3M Attest produce a combination pack that includes a biological indicator and a chemical integrator. The 3M Attest Rapid 5 Steam Plus Test Pack 41382 is specifically designed to present a resistant challenge to the steam sterilization process as does the AAMI 16 towel pack. The 3M Attest Rapid 5 Steam Plus Test Pack 41382 is a biological indicator test pack that contains a 3M Attest 1292 Rapid Readout Biological Indicator and a 3M Comply SteriGage 1243 Steam Chemical Integrator .

What is the Bowie-Dick Test?
The Bowie-Dick Test is a standardized test that ensures all air is removed from vacuum sterilizer and the that the sterilizer does not have any air leaks. The Bowie-Dick Test is not a biological indicator. The Bowie-Dick Test should be performed every day before the sterilizer is used. The Bowie-Dick Test provides a color indicator is the sterilizer passes or fails the test. The Bowie-Dick Test does not provide any information regarding the specific cause of the failed test.
Bowie-Dick Test packs are usually sold in packs and some like the 3M Comply Bowie-Dick Test 00135LF have an early warning sheet that detects potential issues before they appear on the test sheet.

For more information on choosing the best biological indicators and other sterilization supplies, contact USA Medical and Surgical Supplies. Call us toll-free at 1-866-561-2380 or send an email on our Contact form.



